Independent of ANY Mutation, the SARS-COVID 19 corona virus quickly proliferates as soon as the virus enters the body. In the meantime, some metabolic and chemical changes begin to occur in the body before clinical symptoms, lung symptoms and PCR test positivity have yet to occur.
In this process, the virus begins to be excreted in the urine. As the burden of the virus increases in the body, both the virus and certain substances in the urine due to metabolic changes begin to be excreted intensively.
The test is conducted in a laboratory using a specially prepared reactant (CEC ) that reacts with the substances found on the surface of the virus and with the substances excreted in the urine and the sample inside the test tube foams the urine.
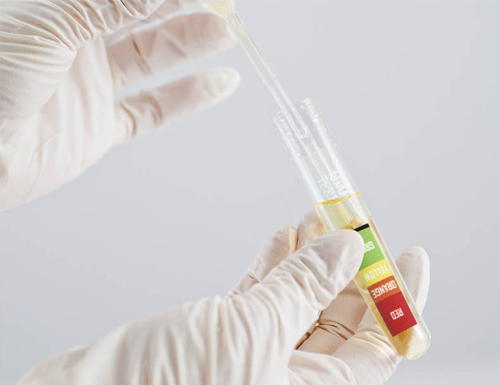
The rate of foaming varies in direct proportion to the virus load in the body. For people who have any viral load, less foam or more foam will be produced in the test tube, providing a VISUAL volume level indicator.
As a result of laboratory studies conducted on more than 500 patients and more than 1000 healthy individuals diagnosed with COVID-19, the amount of foam was converted into a color scale on the test tube. Validation studies have shown up to 98% correct results (Positive or Negative COVID-19 viral load).
GREEN ZONE
This indicates no viral load in the urine. Favorable clinical picture.
The green zone of the foaming test result of the patients who were ALREADY diagnosed with Covid-19 positive and HAVE ALREADY started to be treated, is interpreted as showing that the treatment process was successful and the virus load in the patient body has disappeared.
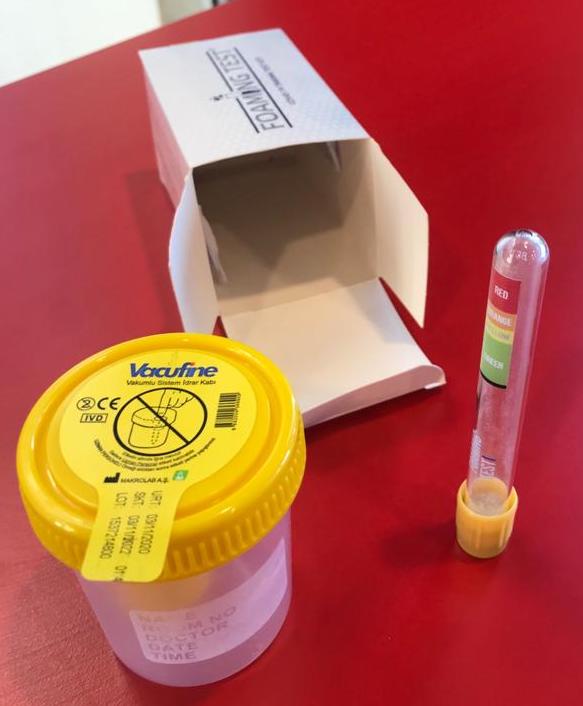
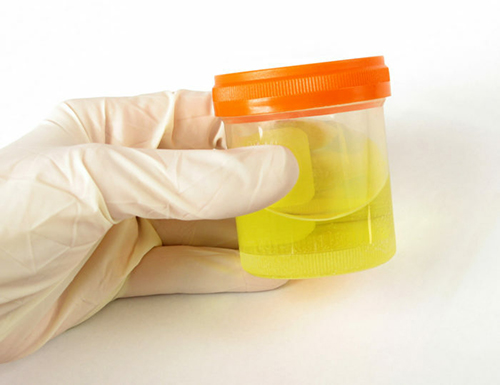
2. Fill in the test tube with Urine Sample until the black line (the Vacutainer will fill up by itself to the correct level if it was Vacuum prepared).
Anyone, anywhere can do all stages of the test under hygienic conditions. Depending on your location and Test Result, conserve or dispose of the Samples in a Safe Manner. ALWAYS Follow local Health Department regulations. NOTE: The 98% Probability Diagnostics is the result of Validation testing, available to Customers and Research Organisations. Here is the FULL PROCEDURE: